- HOME
- NEWS
- RCAST Report
- Discovery of a thermogenic phospho-switch that controls inducible thermogenic fat cells
Discovery of a thermogenic phospho-switch that controls inducible thermogenic fat cells
- Research News
October 24, 2022
A potential therapeutic target for obesity and type 2 diabetes by promoting fat-burning cells by orchestrating chromatin structure and co-activator recruitment
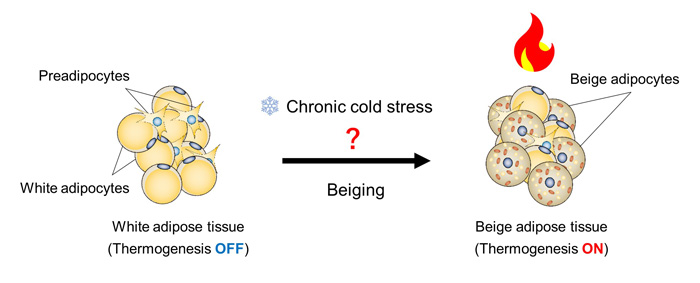
Obesity caused by over-nutrition can lead to lifestyle-related diseases such as type 2 diabetes. In recent years, obesity has become widespread throughout society, and the development of methods to prevent and treat it has become an urgent issue. Thermostatic animals, including humans, have a mechanism that changes white adipose tissue, which stores and supplies energy, into beige adipocytes that burn fat and produce heat when exposed to cold environments for a long time (Figure 1). Since beige adipocytes actively burn fat, induction of beige adipocytes is attracting attention as a novel strategy for the treatment and prevention of obesity.
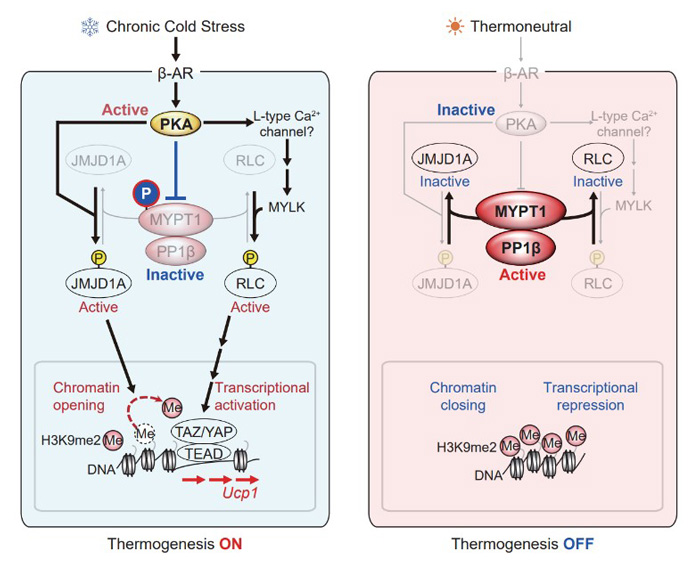
A research group led by Professor Juro Sakai at Tohoku University Graduate School of Medicine / Research Center for Advanced Science and Technology, University of Tokyo, has performed an epigenomic proteomic analysis and found that protein phosphatase (MYPT1-PP1β) normally regulates both epigenome rewriting enzymes and transcriptional regulation to prevent beige fat We found that MYPT1-PP1β suppresses the induction of beige adipocytes. On the other hand, under cold stimulation, protein phosphatase (PKA) inhibits MYPT1-PP1β in activated cells and induces beige adipocytes (Figure 2). In addition, mice lacking adipose tissue-specific MYPT1 were found to be less likely to gain weight and develop impaired glucose tolerance even when fed a high-fat carbohydrate diet. The results of this research are expected to be applied to new therapeutic and preventive methods for obesity and lifestyle-related diseases.
The research results were published online in the international scientific journal Nature Communications on September 29, 2022.
Professor Sakai commented as follows. “In this study, we focused on the phosphorylation of the epigenetic enzyme JMJD1A, which induces beiging by sensing cold stress. It was a challenging attempt to verify whether it is possible to induce a “fat-burning memory” into adipocytes by modulating post-translational modification of an epigenetic enzyme. This presents the possibility of development of a new anti-obesity drug by enhancing the function of epigenomic enzymes and controlling epigenetic changes at specific loci, and has great potential in life sciences and in the prevention and treatment of multifactorial diseases.”
This research was supported by Grant-in-Aid for Scientific Research (S), MEXT, Japan, "Elucidation of the onset of lifestyle-related diseases by environmental factors and epigenetic memory", Research Activity Start Support "Elucidation of the mechanism of adipocyte browning by identifying the phosphorylation regulatory protein of histone demethylases", Japan Agency for Medical Research and Development, Innovative Advanced Research and Development Support Program (AMED-CREST) "Elucidation of Life Phenomena in Early Life Stages for the Improvement of Health and Medical Care" in the research and development area, "Elucidation of Life Environment Memory in Early Life Stages that Works for the Prevention of Lifestyle-related Diseases", and other research activities supported by AMED-CREST.
-
Figure 1. long-term cold environment induces beige adipocytes in white adipose tissue, which are responsible for chronic heat production. ©2022 Juro Sakai
-
Figure 2: How cold causes white fat cells to become beige fat cells.
The circled 'P' in the figure represents protein phosphorylation and 'Me' represents dimethylation of the 9th lysine of the histone H3 protein. Under chronic cold-stimulated conditions, MYPT1-PP1β is suppressed, JMJD1A and RLC are activated, and heat production-related genes are turned on. Under non-chill-stimulated conditions, MYPT1-PP1β is activated, JMJD1A and RLC are suppressed, and heat production-related genes are turned OFF. ©2022 Juro Sakai
【Paper】
- Author:
- Hiroki Takahashi*, Ge Yang*, Takeshi Yoneshiro, Yohei Abe, Ryo Ito, Chaoran Yang, Junna Nakazono, Mayumi Okamoto-Katsuyama, Aoi Uchida, Makoto Arai, Hitomi Jin, Hyunmi Choi, Myagmar Tumenjargal, Shiyu Xie, Ji Zhang, Hina Sagae, Yanan Zhao, Rei Yamaguchi, Yu Nomura, Yuichi Shimizu, Kaito Yamada, Satoshi Yasuda, Hiroshi Kimura, Toshiya Tanaka, Youichiro Wada, Tatsuhiko Kodama, Hiroyuki Aburatani, Min-Sheng Zhu, Takeshi Inagaki, Timothy F. Osborne, Takeshi Kawamura, Yasushi Ishihama, Yoshihiro Matsumura#, Juro Sakai#(*first author, #corresponding author)
- Title:
- MYPT1-PP1β phosphatase negatively regulates both chromatin landscape and co-activator recruitment for beige adipogenesis
- Journal:
- Nature Communications
- Publication Date:
- 2022/9/29
- DOI:
- 10.1038/s41467-022-33363-0
- URL:
- https://www.nature.com/articles/s41467-022-33363-0
【Research Contact】
Juro Sakai, Professor
Tags

